Natural Highs? Exploring the Endocannabinoid System
By: Srinivas Sowmiyanarayanan
Marijuana is the most used illicit substance in the United States (CDC). Cannabis products and their usage are more and more relevant as they are being legalized in many states throughout the United States. Cannabis produces various effects on the human body, such as creating overall pleasure, altering senses, and stimulating appetite, to name a few (NIDA, 2021). From a biological standpoint, this raises an important question: how do the compounds within cannabis plants interact with our cells? Like other drugs, cannabis plants contain chemical compounds, cannabinoids such as tetrahydrocannabinol (THC) and cannabidiol (CBD), that “bind to G protein–coupled cannabinoid receptors CB1 and CB2” on our cells (Chayasirisobhon, 2021,). G protein-coupled receptors are a type of chemical receptor that are found on cell membranes and are involved with cell communication. The cannabinoid receptors are found throughout the body but CB1 is especially prevalent in the cells of the brain and spinal cord (Vlachou & Panagis, 2014). Since our cells naturally have receptors that bind to cannabinoids, we also have endogenous analogs to cannabinoids. Identifying these cannabinoid receptors points towards the existence of natural ligands, including “N-arachidonoyl-ethanolamine (AEA; anandamide) and 2-arachidonoylglycerol (2-AG)” (Zou & Kumar, 2018).
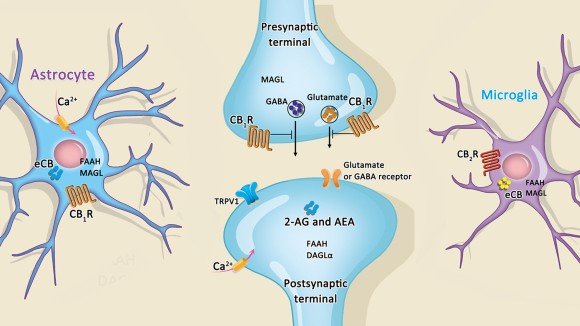
Figure 1: A diagram of a synapse and the corresponding pre- and post-synaptic terminals showing how the cannabinoid receptor CB1 affects neuronal signaling.
Researchers have categorized the cannabinoid receptors along with their corresponding ligands into their own signaling system: the endocannabinoid system.
The endocannabinoid system has deservedly received scientific interest since it is involved with so many biological processes including appetite, pain regulation, and mental illness.
Currently, a synthetic form of THC known as dronabinol is approved by the FDA to treat nausea and improve appetite for cancer patients (Badowski, 2017). Pacher & Kunos showed in their study that the endocannabinoid system affects multiple pathways related to hunger and feeding by comparing the food consumption of mice with and without the CB1 receptor (2006). This study also highlights that cannabinoids affect appetite and feeding through not only mechanisms that affect appetite directly, but also mechanisms that affect behaviors, further emphasizing the ubiquitous nature of the endocannabinoid system in humans (Pacher & Kunos, 2006). Badowski’s review of oral cannabinoids, such as dronabinol, and medical marijuana demonstrates that both show efficacy in reducing nausea, but notes that there are variations in the efficacy between the sources of cannabinoids, such as medical marijuana and dronabinol, and that both have side effects associated with cannabis use such as motor impairment and a psychological high (2017).
Another therapeutic use for cannabinoids that is not yet federally approved is pain management. The evidence underlying this use is not as conclusive as the evidence for cannabinoids in treating nausea and vomiting. However, some research has shown that “smoked marijuana was found to be better than placebo in relieving [neuropathic] pain” (Kramer, 2015). Another review corroborates this with other researchers’ findings by stating that “THC or Sativex reduced neuropathic pain in patients with traumatic nerve injury or multiple sclerosis in randomized, double-blind, placebo-controlled, crossover trials'' (Pacher & Kunos, 2006). They note that cannabinoids “exert their antinociceptive effects by complex mechanisms involving effects on the central nervous system” (Pacher & Kunos, 2006). Further research into pain management with cannabinoids needs to be done to concretely determine their efficacy and pharmacological mechanism(s) of action.
With an abundance of cannabinoid receptors in the cells of the brain, it should be no surprise that cannabinoids are involved with mental processes including mental illness.
The THC in cannabis is responsible for its psychoactive effects, which range from “euphoria” to “anxiety” or “acute psychosis” (NIDA, 2021).
Figure 2: How cannabinoids affect different regions of the brain.
A study by Vlachou and Panagis (2014) highlights that “the endocannabinoid system has been implicated in the pathophysiology of neurological and psychiatric disorders, such as … drug addiction, depression, … and schizophrenia.” These findings raise important implications for cannabinoids as a drug class: how can we target specific symptoms/diseases while preserving cognitive processes? This issue is further exacerbated by the fact that in non-clinical settings, one of marijuana’s recreational uses is “to cope with anxiety, depression, and other psychiatric symptoms” yet those “who report self-medicating with cannabis to reduce psychological distress have been found to have higher rates of generalized anxiety, panic disorder, and obsessive-compulsive disorder” (Wallis et al., 2022). This research importantly highlights that cannabinoids are not a cure-all as many proponents might claim; using cannabinoids therapeutically involves interacting with the human body’s delicate biochemistry. It is clear that the endocannabinoid system has wide-reaching implications within the human body and necessitates further medical research to fully understand the role that it plays before we can expand the therapeutic usage of cannabinoids.
References
Badowski M. E. (2017). A review of oral cannabinoids and medical marijuana for the treatment of chemotherapy-induced nausea and vomiting: a focus on pharmacokinetic variability and pharmacodynamics. Cancer Chemotherapy and Pharmacology, 80(3), 441–449. https://doi.org/10.1007/s00280-017-3387-5
CDC. (2021, June 8). Marijuana and public health: data and statistics. Retrieved February 12, 2023, from https://www.cdc.gov/marijuana/data-statistics.htm
Chayasirisobhon, S. (2021). Mechanisms of action and pharmacokinetics of cannabis. The Permanente Journal, 25(1), 1–3. https://doi.org/10.7812/TPP/19.200
Kramer, J. L. (2015). Medical marijuana for cancer. CA: A Cancer Journal for Clinicians, 65(2), 109–122. https://doi.org/10.3322/caac.21260
NIDA. (2021, April 19). What are marijuana's effects?. Retrieved February 12, 2023, from https://nida.nih.gov/publications/research-reports/marijuana/what-are-marijuana-effects
Pacher, P., Bátkai, S., & Kunos, G. (2006). The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacological Reviews, 58(3), 389–462. https://doi.org/10.1124/pr.58.3.2
Vlachou, S., & Panagis, G. (2014). Regulation of brain reward by the endocannabinoid system: A critical review of behavioral studies in animals. Current Pharmaceutical Design, 20(13), 2072–2088. https://www.eurekaselect.com/article/53098
Wallis, D., Coatsworth, J. D., Mennis, J., Riggs, N. R., Zaharakis, N., Russell, M. A., Brown, A. R., Rayburn, S., Radford, A., Hale, C., & Mason, M. J. (2022). Predicting self-medication with cannabis in young adults with hazardous cannabis use. International Journal of Environmental Research and Public Health, 19(3), 1850. https://doi.org/10.3390/ijerph19031850
Zou, S., & Kumar, U. (2018). Cannabinoid receptors and the endocannabinoid system: Signaling and function in the central nervous system. International Journal of Molecular Sciences, 19(3), 833. https://doi.org/10.3390/ijms19030833
Images
Acta Pharmacologica Sinica. (2019). Cannabinoid receptors. Cannabis, cannabinoid receptors and endocannabinoid system. Retrieved February 12, 2023, from https://www.nature.com/collections/hccdeebaid/content/article.
NIDA. (2020). Effects of marijuana on the brain. How does marijuana produce its effects? Retrieved February 12, 2023, from https://nida.nih.gov/publications/research-reports/marijuana/how-does-marijuana-produce-its-effects.