Unraveling the Role of Gut Microbiota in Cancer: Harnessing Probiotics for Anticancer Therapies
By: Sanjit Hajgude
The human microbiota has been implicated in the development of several diseases, including diabetes and neurodegenerative diseases. Growing recognition of these microorganisms has led to the discovery of the gut microbiota’s complex role in the development of cancer and anticancer drug efficacy (Cheng WY et al., 2018). This article explores the relationship between gut microbiota and anticancer therapies, focusing specifically on the impact of probiotics on cancer treatment strategies.
The gut alone houses more than 100 trillion microbial cells which may influence physiological processes by promoting, mitigating, or having no direct effects on tumorigenesis (Cotter & Guinane, 2013). Some gut bacteria such as Helicobacter pylori intensify cancerous growth through “procarcinogenic signaling pathways,” while others including Streptococcus thermophilus suppress tumors by “producing anticancer metabolites” (Huang et al., 2022). These effects reflect gut dysbiosis—an imbalance between gut microbiota and the human host that manifests both positive and negative alterations to cancer development. Furthermore, mounting evidence suggests that gut bacteria are involved in the modulation of drug therapies by means of processes like immunomodulation—regulation of macrophages and T-cell responses—as well as enzymatic degradation (Ağagündüz et al., 2023).
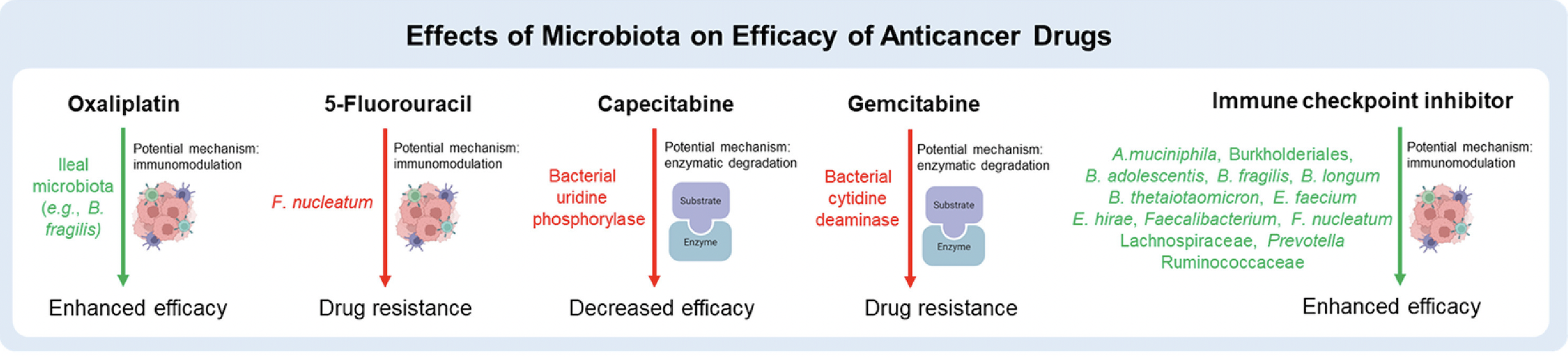
Figure 1. Effects of microbiota on the efficacy of five anticancer drugs; green indicates increased drug efficacy, while red indicates decreased drug efficacy.
Uncovering these links has led to gut microbiota being a highly desirable target for predicting anticancer drug response and improving the efficacy of drug mechanisms.
It is critical to understand both the beneficial and harmful effects of the gut microbiota in relation to cancer development and anticancer treatments to generate potential clinical applications that are successful.
Recent studies have indicated that supplemental probiotics, including natural and genetically modified bacteria, show great potential for improving the efficacy of certain anticancer drugs. Probiotics utilize their anticancer metabolic properties, including SCFAs (short-chain fatty acids), to inhibit histone deactlayzes of cancer cells; this, in turn, stimulates apoptosis of cancer cells and induces other antitumor effects (Akbar et al., 2022). When paired with anticancer drugs used in chemotherapy and immunotherapy, probiotics can influence anti-programmed death ligands and therapeutic activity. Immune checkpoint Inhibitors (ICI) therapy, a treatment for cancer, can cause severe inflammatory effects, but probiotics have been shown to alleviate overall inflammation in patients by mechanisms of immunomodulation (Zhao et al., 2023). A clinical study showcased that the “oral administration of L. reuteri therapeutically inhibited the development and progression of colitis, thus ameliorating the loss of body weight and inflammatory status induced” by ICIs (Wang et al., 2019). By inhibiting group-3 innate lymphoid cells, a type of immune cell, L. reuteri was able to decrease overall inflammation caused by colitis in ICI treatment.
While probiotics indicate positive results in reducing tumorigenesis, improving the efficacy of anticancer therapies, and alleviating side effects of anticancer drugs, several complications still remain that hinder their adoption as an ideal supplement in current oncopharmacology. One of these complications includes the multiresponse behavior of some bacterial strains in modulating the efficacy of different anticancer drugs. For example, Fusobacterium nucleatum strains may reduce the efficacy of 5-Fluorouracil, a chemotherapy drug, in some patients, but may also promote the efficacy of PD-L1 blockades in other patients, improving anti-tumor immune responses (Huang et al., 2022).
The duality of certain bacteria poses a problem in administering probiotics to cancer patients, as predicting a universal outcome in cancerogenesis becomes difficult.
In addition to adverse toxicity from certain bacteria, probiotics face barriers in clinical trials and related research. Many probiotic candidates such as the orally administered bacterial consortium, VE800, have failed to advance to the next stages of clinical trials because of indeterminate data or observed response rates not meeting prespecified criteria (Davar et al., 2020). The variability of gut microbiota samples and the diverse effects of these microorganisms on patients makes comprehending the mechanistic properties of microbiota difficult.
Figure 2. Upward trend in the number of clinical trials over time on microbiota (gray), probiotics (blue), and prebiotics (orange).
However, new methods have developed in the past decade that may allow researchers to profile microbes. By using techniques like Microbe-seq, “a high-throughput method that yields the genomes of individual microbes from complex microbial communities,” scientists can better capture precise mechanisms of targeted microbes. (Zheng, et al., 2022).
It is clear that the intricate relationship between human gut microbiota and cancer development has extensive implications within the human body, and understanding the mechanistic properties of these microorganisms will allow researchers to adopt probiotics as a valid candidate in oncopharmacology in the near future.
References
Akbar, N., Khan, N. A., Muhammad, J. S., & Siddiqui, R. (2022). The role of gut microbiome in cancer genesis and cancer prevention. Health Sciences Review, 2, 100010. https://doi.org/10.1016/j.hsr.2021.100010
Ağagündüz, D., Cocozza, E., Cemali, Ö., Bayazıt, A. D., Nanì, M. F., Cerqua, I., Morgillo, F., Saygılı, S. K., Berni Canani, R., Amero, P., & Capasso, R. (2023). Understanding the role of the gut microbiome in gastrointestinal cancer: A Review. Frontiers in Pharmacology, 14. https://doi.org/10.3389/fphar.2023.1130562
Cheng, W. Y., Wu, C.-Y., & Yu, J. (2020). The role of gut microbiota in cancer treatment: Friend or foe? Gut, 69(10), 1867–1876. https://doi.org/10.1136/gutjnl-2020-321153
Davar, D., Wang, J. S., Cecchini, M., Wainberg, Z., Gutierrez, M., Turk, A., Szabady, R., Norman, J., Olle, B., Roberts, B., & Bobilev, D. (2020). Abstract CT246: Consortium-IO: A safety and efficacy study of VE800 in combination with nivolumab in previously treated patients with select advanced metastatic cancers. Cancer Research, 80(16_Supplement). https://doi.org/10.1158/1538-7445.am2020-ct246
Guinane, C. M., & Cotter, P. D. (2013). Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ. Therapeutic advances in gastroenterology, 6(4), 295–308. https://doi.org/10.1177/1756283X13482996
Huang, J., Liu, W., Kang, W., He, Y., Yang, R., Mou, X., & Zhao, W. (2022). Effects of microbiota on anticancer drugs: Current knowledge and potential applications. EBioMedicine, 83, 104197. https://doi.org/10.1016/j.ebiom.2022.104197
Wang, T., Zheng, N., Luo, Q., Jiang, L., He, B., Yuan, X., & Shen, L. (2019). Probiotics lactobacillus reuteri abrogates immune checkpoint blockade-associated colitis by inhibiting group 3 innate lymphoid cells. Frontiers in Immunology, 10. https://doi.org/10.3389/fimmu.2019.01235
Zhao, L.-Y., Mei, J.-X., Yu, G., Lei, L., Zhang, W.-H., Liu, K., Chen, X.-L., Kołat, D., Yang, K., & Hu, J.-K. (2023). Role of the gut microbiota in anticancer therapy: From molecular mechanisms to clinical applications. Signal Transduction and Targeted Therapy, 8(1). https://doi.org/10.1038/s41392-023-01406-7
Zheng, W., Zhao, S., Yin, Y., Zhang, H., Needham, D. M., Evans, E. D., Dai, C. L., Lu, P. J., Alm, E. J., & Weitz, D. A. (2022). High-throughput, single-microbe genomics with strain resolution, applied to a human gut microbiome. Science, 376(6597). https://doi.org/10.1126/science.abm1483
Images:
Dronkers, T. M. G., Ouwehand, A. C., & Rijkers, G. T. (2020). Development in the number of clinical trials over time. Heliyon, 6(7). https://doi.org/10.1016/j.heliyon.2020.e04467
Huang, J., Liu, W., Kang, W., He, Y., Yang, R., Mou, X., & Zhao, W. (2022). Effects of microbiota on the efficacy and toxicity of anticancer drugs [Illustration]. eBioMedicine, 83, 10419. https://doi.org/10.1016/j.ebiom.2022.104197